Pharmaceutical Packaging Seal integrity testing, Micro Vacuum Decay Leak tester, ASTM F2338, EP/GMP/FDA
PKT-MF2338
LabGeni is indicated to manufacturing and delivering machinery of the highest quality, providing top performance , efficiency and reliability for your packaging and inspection needs.
Product info
LabGeni is indicated to manufacturing and delivering machinery of the highest quality, providing top performance , efficiency and reliability for your packaging and inspection needs.PKT-MF2338 Packaging Leak Tester is known as the leak detection experts in virtually every major market, to the medical industry, including other medical devices. As part of the analysis and tester production of Companies, PKT-MF2338 packaging leak tester works with professional engineer to help customers compete and win with turnkey solutions they can trust in an Industry world increasingly defined by data.
PKT-MF2338 packaging leak tester combines its family of leak detection instruments and its extensive experience in pressure decay, vacuum decay, mass flow, and helium leak testing in large volume leak-testing, and in data management for traceability, test optimization, and root-cause analysis. Together, these partners can customize for any manufacturer in almost any vertical the right leak test for the job and employ the data-driven insight that ensures quick set-up and reliable performance from day one.
It is suitable for sealing integrity testing of pharmaceutical packaging to prevent moisture, oxygen, and microorganisms from contaminating products. Medicines: bottled, bagged, boxed, ampoules, vials, cartridges, prefilled needles (PFS), (BFS), (FFS), etc.
Spray cans: plunger spray cans; bag-lined spray cans; "energy jacket" spray cans; flexible tube spray cans.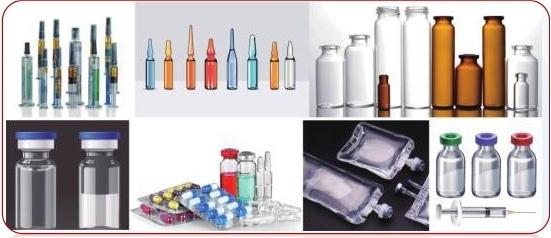
Accurate Results
Our PKT-MF2338 Packaging Leak Testing System use vacuum testing to deliver highly accurate results. With the ability to detect leaks of just a few microns, these systems produce quantitative results with logging for long-term trend analysis and data traceability.
Non-Destructive Testing
Non-destructive testing is one of the features of several of our systems including the PKT-MF2338 Packaging Leak Testing System. Non-destructive testing reduces waste by allowing products to be tested and passing products to be returned to the packing line. With non-destructive testing, fewer products are damaged with the intent of quality testing.
Recall Reduction
LabGeni’s PKT-MF2338 Packaging Leak Tester help businesses to cut costs by reducing the risk of a product recall. These testing systems ensure the integrity of the packaging for a product. Damaged or improperly sealed packaging is often the source of contamination in many recalls. Using leak testing methods can readily identify packaging issues for immediate resolution.
We ship our PKT-MF2338 Packaging Leak Tester to customers around the globe. With fast and friendly customer support and sales assistance, we can ensure that you will be satisfied with the performance and durability of our systems.
ü The LabGeni’s PKT-MF2338 Packaging Leak Tester tests various of packages for leaks, and gives go/no-go answers independent of the operator. It can find holes as small as 2μm for smaller packages and also is applicable for the largest packages. It’s used to test medical bandages, pharmaceuticals, and the list goes on
ü A unique flexible test chamber ensures good packages are left in original condition after testing and put straight back onto the production line. Which decreases the return-on-investment time. It requires packages with a “headspace” of air (or MAP gases) and generally dry contents.
ü LabGeni’s PKT-MF2338 Packaging Leak Tester has a simple change testing chamber allows a larger range of packages to be tested on the machine. Items are placed manually, and the handle is closed to initiate a fully automatic test sequence. Results are displayed with Pass or Fail lamps, along with a quantitative measure of the leakage rate.
How does vacuum decay testing work?
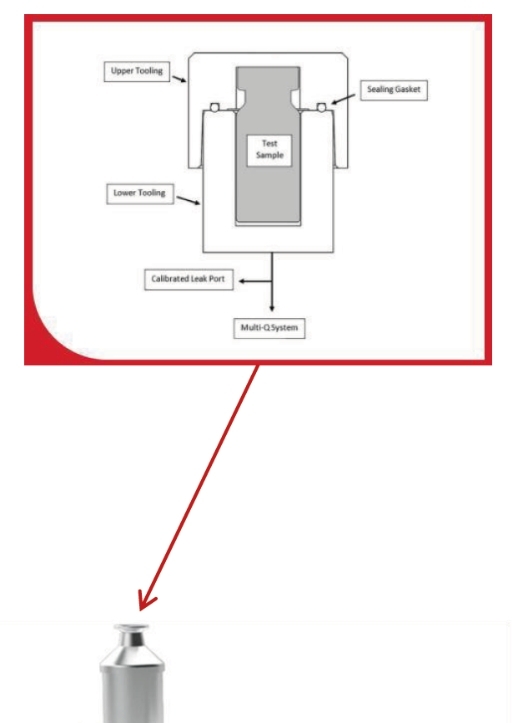
To conduct a vacuum decay test, a container is placed in a tightly fitted chamber which is evacuated to a predetermined level of vacuum. After reaching the pre-set vacuum, a sensor measures the vacuum level over a predetermined time. If the integrity of the container under test has been compromised (it has a leak), there will be a rise in pressure measured inside the chamber.
This will result in a) a gross leak, if the vacuum doesn’t reach the pre-set vacuum level; b) a medium leak, if the vacuum level drops below a pre-set vacuum level during the test or c) a decay or micron leak, if the vacuum level exceeds the decay limit during the test time.
Vacuum Decay Test Advantages
The vacuum decay leak testing method can provide numerous benefits that can provide quality assurance for sealed packages. Some of the biggest advantages include:
- Determines a leak rate based on pressure or vacuum
- Can calibrate to a volumetric flow
- Sensitive enough to detect very small leaks
- Can test in pressure or vacuum environments
- Can report pressure or flow loss (psig or scc/min)
- Provides a simple and highly effective leak test method
Vacuum Decay Test (Cont.)
Testing a complete pressure vessel allows including the couplers and adapters into leak test. The procedure is the same as described with the difference that the Permeate port at one side of the vessel is closed, and the vacuum is pulled from the permeate port of the other side. Feed and concentrate ports maybe open.
Features
- Comply with USP 1207, ASTM F2338 standards and FDA standards.
- Semi-automatic detection, suitable for small batch and multi-variety testing.
- Non-destructive non-destructive testing, high accuracy, repeatability, sensitivity.
- The instrument is used for vacuum pressure difference detection.
- The leak rate can be automatically converted into defect aperture μm.
- Database storage of test results for easy quality management.
- Database storage of test results for easy quality management.
- Touch-type man-machine interface, simple and quick operation: after setting/selecting the test program, only need to manually put in/take out the test sample.
Advantage function
- Automatically test the flow rate and change the aperture size during the whole process.
- Automatic leak rate calibration function.
- Equipped with standard leaks (standard positive bottles, with third-party certification).
- Four-level user authority management meets FDA 21CFR PART 11 requirements.
- With audit trail function.
- Split design, the test chamber is located above the host, and various test chambers can be provided according to different product types.
We also provide users with supporting services related to tightness testing, including positive bottle production, standard leak rate/annual leak verification, new sample mold customization, sample methodological parameter development and verification, etc.
The test cavity is customized according to customer needs to ensure that the test cavity is fully matched with the customer's product, and rapid and sensitive testing.
SPECIFICATIONS
MODEL | PKT-MF2338 |
Best Sensitivity | 2μm |
Test Pressure Accuracy | ±1% (full scale, best-fit straight line) |
Test Pressure Resolution | Down to ±2 kpa |
Pressure ranges | -0.1~0.2MPa -0.1~0.7MPa -0.1~1.0MPa |
Instrument Dimensions (HxWxD) | 655× 426×410 mm |
Net Weight | 30 kg |
Electronic Testing Record | Yes |
Data Memory | ≤5 years |
Electrical Connection | 230Vac or 115Vac |
Operating Temperature | +10 °C ~ +95 °C (+50 °F ~ +200 °F) |
Storage Temperature | -18 °C to +65 °C (0 °F ~ +150 °F) |
Operating Humidity | 5~ 90 % |
Storage Humidity | 5~ 90 % |
Atmosphere | 700 ~ 1060 hPa |
Signal output | (4-20) mA/RS485/12V Alarm Output |
Connector | Ethernet/ USB/ RS485 |
User Right Control | 4 Level User Control (complying with 21 CFR Part11) |
History Records | ≤5 years capacity |
Printer | YES |
Audit Trail | ≤5 years capacity,Quickly Audit according the time |
Data Backup | Data Derived by USB Flash Disk |
* Please so kindly understand this is precision instrument and should performed at standard laboratory conditions of 26±2 and 65±5% relative humidity.